Research Journal of Applied Sciences, Engineering and Technology
Thermodynamic, Adsorption and Optimization Studies on Carbon Steel in Hydrochloric Acid Environment using Okro Leaf Extract as Corrosion Inhibitor
Research Journal of Applied Sciences, Engineering and Technology 2020 17: 40-53
Cite This ArticleAbstract
This present study seeks to exploit the use of eco-friendly, Okro leaf extract as an inhibitor in mitigating corrosion on carbon steel metal in acidic environment Gravimetric method was used to study the corrosion process. It was revealed that the inhibition efficiency of the extract on carbon steel decreases as temperature increases. This trend confirmed the physical adsorption mechanism for the corrosion process that resulted in a high rate of corrosion of the carbon steel. Activation energy Ea, enthalpy (∆H°), and entropy (∆S°) calculated showed good interactions. The rise in activation energy with inhibitor concentration confirmed the physical (physisorption) adsorption mechanism for the corrosion of a carbon steel surface. The positive value of adsorption enthalpy (∆H°) obtained confirmed the endothermic nature of the corrosion process. The activation entropy (∆S°) values obtained were all negatives which show that the activated complex in the rate-determining step represents association rather than dissociation step. Corrosion inhibition occurred by an attribute of inhibitor molecules adsorption on the surface of carbon steel which is seen to be in conformity with Langmuir, Freundlich, and El-Awady adsorption isotherm models. To obtain the least weight loss of carbon steel, optimization of the process variables affecting the corrosion process was carried out using Response Surface Methodology (RSM). Three parameters were varied viz; inhibitor concentration, contact time and temperature, and their effects on weight loss of carbon steel were established. The obtained data fitted well to the quadratic model which was also validated. The model predicted the lowest weight loss of 0.045 g with the optimal condition of inhibitor concentration of 198.71 ppm, 2.04 hrs of contact time, and 40.29°C of temperature.
Keywords:
Introduction
The consequences of corrosion are numerous, diverse and effects of these on the efficient, safe and reliable operation of equipment or edifices are often more serious than simple loss of a mass of metal. In reality, corrosion can never be stopped but can be hindered to a reasonable level.
Failure of various kinds and the need for expensive replacements may occur even though the amount of metal destroyed is quite small (Umoren et al., 2009). The phenomenon of corrosion mitigation, abatement and inhibition are major scientific issues that should be tackled daily due to the increasing need for metallic materials in the technological advancement of our industries. Owing to harms arising from corrosion that are challenging the chemical and process industries; several methods of controlling corrosion and prevention have been put in place. Some of these methods include; anodic and cathodic protection, lubrication, galvanizing, alloying, coating and the use of organic and inorganic inhibitors. The choice and application of any of these methods was based on their economic factors, efficiency and nature of the corrosive environment (Njoku 1998). The use of inhibitor is one of the most practical methods for protection against corrosion in corrosive environments. Inhibitors are chemicals that directly or indirectly coat a film on a metal surface to protect it from its environment. Generally, inhibitors are absorbed by the metal surface from a solution or dispersed, but some are applied directly as coatings. However, the dissolution of metal can be suppressed by the action of adsorptive inhibitors which may prevent the adsorption of the aggressive ions and by the formation of a more resistant film on the metallic surface (El Maghraby 2009). Historically, most of the known corrosion inhibitors are synthetic chemicals (heavy metal), expensive and very hazardous to environment.
Therefore, it is desirable to source for environmentally safe inhibitors (Paul et al., 2012). In view of these, researches had been undertaken on the development of an ecofriendly natural inhibitors and one of such is Okro leaf (Abelmoschus esculentus). The Okro leaf phytochemical is rich in tannins, steroids, flavonoids, phenols and terpenoids (Dheba et al., 2017). These phytochemical constituents especially tannins have an array of hydroxyl and carboxyl groups through which the molecules can adsorbed on corroding metallic surfaces thus inhibiting the corrosion process on the carbon steel (Oki et al., 2011).
Optimization and modeling is of great importance in any process as it improves the yield (Adepoju and Eyiobi, 2016). The use of a unity variable method for modeling and optimization is now becoming outdated because it does not show any interaction between the other variables considered in the process (Seramen et al., 2010). Response Surface Methodology (RSM) is a method of optimization which consists of experimental design, analysis and modeling through the partial fitting of regression from the experimental factors (Wang et al., 2011). RSM has the ability to link many variables at a time and display the mutual interaction on the yield of a route, it also facilitate reduction in the amount of experimental runs required to provide adequate evidence for results that are acceptable statistically. Extract from leaves has been studied as a potential for corrosion inhibitor but no research has been conducted on the use of Okro leaf extract as a corrosion inhibitor. Likewise, RSM has not been reported on corrosion inhibition of carbon steel using Okro leaf extract.
This present work studied the Okro leaf extract as an inhibitor, kinetics and mechanism of adsorption and also optimized the process variables already reported in literature affecting corrosion process (Yawas, 2005). In order to minimize the weight loss of the carbon steel, RSM was used to know the effects of three factors (temperature, concentrations and contact time) and their reciprocal effect on rate of corrosion on carbon steel metal. The work established the process condition in a view to achieving minimum weight loss on carbon steel which could be applied to local and industrial corrosion treatment.
Materials And Methods
The okro leaves (Abelmoschus esculentus) were collected from Agbahro community located in Effurun, Delta State, Nigeria. Soxhlet extractor apparatus (Techmel and Techmel, USA), oven (Gallenkamph 2), PGW752i weighing balance with resolution of 0.001 mg were used for the study.
Okro leaf extract preparation: The Okro leaves (Abelmoschus esculentus) were washed thoroughly with running water to remove unwanted materials. The washed samples were dried in open air for 4 days and grinded to a particle seize of 0.143 µm. The sample was stored in a desiccator before used. 25 g of the dried alligator pepper pods powder was transferred into a 500 mL round-bottom flask and 300 mL of 70% ethanol. A reflux condenser was then connected to the flask and cold water was allowed to flow through the condenser for better reduction of solvent losses. The set-up was placed on a heating mantle and the Okro leaf extract was extracted exhaustively by heating the solution under reflux at 78°C. Rotary evaporator (model R-210) was used to remove the remaining solvent (ethanol) from the extract.
Procedure of the experiment: The gravimetric or weight loss method was used. The carbon steel was mechanically polished with silicon carbide abrasive paper, degreased with ethanol, washed in distilled water and dried in acetone. Each carbon steel coupon was sized 40mm × 20mm × 2mm. Before polishing, a hole of 0.1cm was drilled on each coupon. The coupon was suspended with the aid of a nylon thread in a 100mL beaker with 100 mL of 1.5M HCl at different inhibitor concentrations.
The mechanism of inhibition and thermodynamic parameters were studied at 308, 318, 328, 338 and 348K temperature at contact time of 7h. Each of the carbon steel metal coupons after the corrosion process was dipped in both distilled water and ethanol solutions. This was scrubbed to remove any remaining residual inhibitor concentration and HCl. Thereafter, the coupon was then washed thoroughly with washing liquor, rinsed with distilled water and later dried in acetone before been reweighed.
Determination of weight loss: The weight loss of the mild steel coupon was determined using Eq. (1):
where,
Determination of inhibitor efficiency: The efficiency of corrosion inhibition was obtained using the equation below:
where,
Optimization studies on rate of corrosion on carbon steel: Optimization of the process variables influencing the rate of corrosion was carried out using Response Surface Methodology (RSM). Three variables were varied viz; concentration of Okro leaf extract (inhibitor), temperature and contact time and their effects on weight loss of the carbon steel was investigated. 17 experiments runs were generated using the Box Behnken Design (BBD). The model fitness was evaluated using test of significance and Analysis of Variance (ANOVA). The selected factors concentration of inhibitor, contact time and temperature represent X1, X2 and X3 respectively. This is shown in Table 1.
The coefficient of the polynomial model was determined using the multiple regressions as shown in Eq. (3):
where,
(Omoruwou et al., 2017; Betiku and Adesina, 2013). Design Expert 7.00 software was used to design and analyze the data from the experiment. This is a statistical software package that does design of experiments, comparative tests and optimization of the process variables. It is also used to study the parameters on the yield of a process using the graphical tool.
| Variables | Symbol | Low factor (-1) | Mid-point factor (0) | High factor (+1) |
|---|---|---|---|---|
| Concentration of inhibitor, (ppm) | X1 | 50 | 150 | 250 |
| Contact time, (hr) | X2 | 2 | 7 | 12 |
| Temperature, (°C) | X3 | 35 | 45 | 55 |
Results And Discussion
As evidenced in Table 2, the preponderance of tannin, flavonoids, steroids, phenols and terpenoids in the Okro leaf Extract can be said to enhance the corrosion inhibition of carbon steel in acidic environment studied. The presence of these compounds has been reported to promote the corrosion inhibition of mild steel in aggressive acidic media (Umoren et al., 2006). This also corroborates the work of Prithiba et al., (2014), Owate et al., (2014) and Nwigbo et al., (2012).
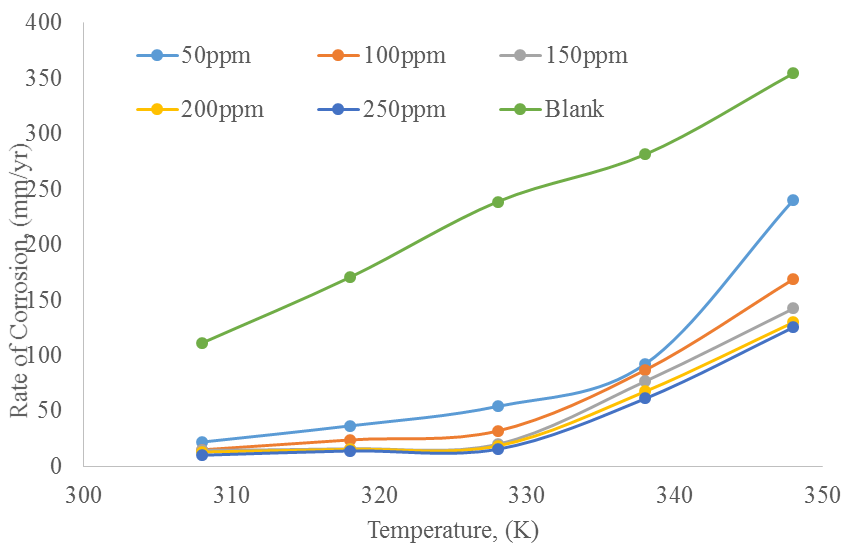
Effect of temperature of corrosion rate of carbon steel: Figure 1 present the effect of temperature on rate of corrosion of carbon steel. It can be seen that the rate of corrosion is higher in medium without inhibitor in comparison to the one that has inhibitor. Increased in temperature led to increase in corrosion rate of the carbon steel due to the fact that at higher temperature the process of corrosion occurred rapidly due to weakening of the adsorption capacity of the inhibitor due to the hot-movement of inhibitor molecules from the metal surface. The obtained result is in agreement with reports of (Shivakumar and Mohana, 2012; Norzila and Ishak, 2015).
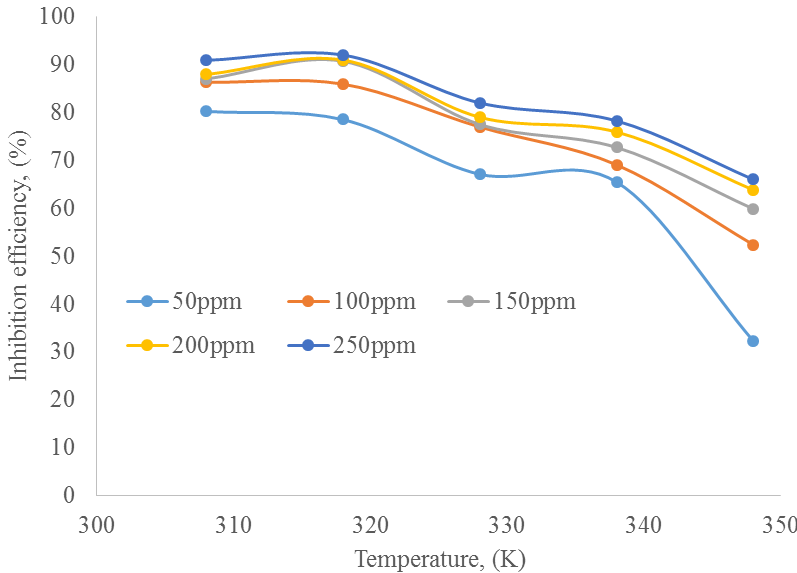
Temperature effect on inhibition efficiency: The inhibition efficiency of the inhibitor for the carbon steel decreases with increasing temperature, a trend that confirm physical adsorption mechanism for the corrosion process that is caused by desorption of adsorbed inhibitor as a result of higher rate of hydrogen evolution due to increased agitation of solution brought by higher temperature. This is similar to the result obtained by Abd El-hameed (2011) (Fig. 2).
Thermodynamics and adsorption studies: Thermodynamic properties such as Activation Energy (Ea), Enthalpy (∆H°) and Entropy of Activation (∆S°) were undertaken so as to ascertain the mechanism of adsorption process involved in the carbon steel corrosion process. In order to calculate the thermodynamics parameters like enthalpy (∆Hads) and entropy (∆Sads) of corrosion process in the presence and absence of Okro leaf extract inhibitor in hydrochloric acid solution the transition state theory equation given by Eq. (4) was used (Alhaffar et al., 2018; Ogoke et al., 2009):
where, h is the Planck's constant
where,
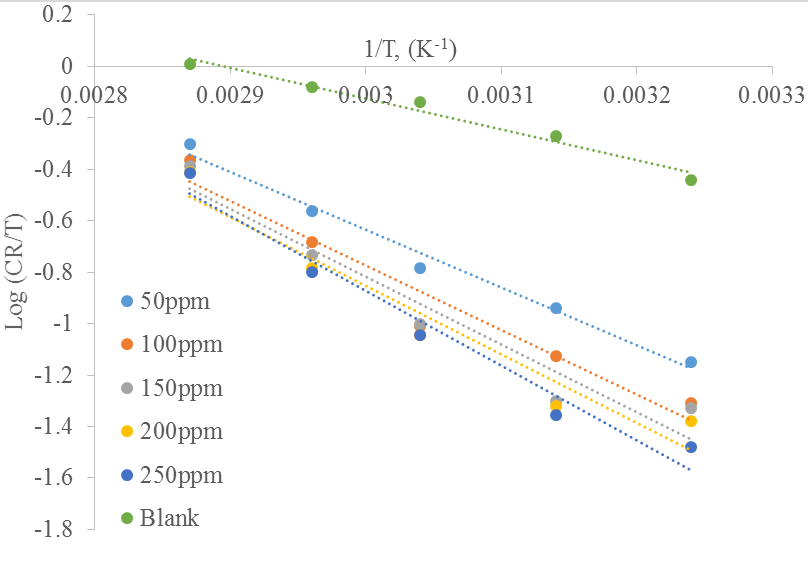
The Arrhenius plot of log
Table 3 shows the value of activation parameters obtained in this study. It can be seen that the values of corrosion enthalpy of adsorption
This implied to mean that the activated complex in the rate determining step represents association rather than dissociation step, indicating that during the adsorption process, a decrease in the degree of orderliness takes place when moving to the activated complex from the reactants with increased in inhibitor concentration, the order of activated complex entangled in the rate determining step of the corrosion reaction becomes more dissociated (Alhaffar et al., 2018). Hence, disorderliness is decreased as reactants are transformed to activated complex.
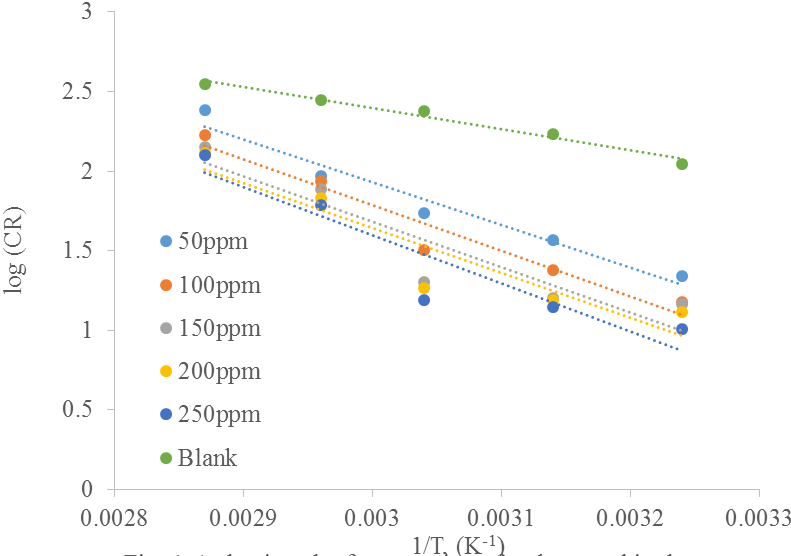
The value of Ea (activation energy) in blank solution is 25.62 kJ/mol and rise as inhibitor concentration is increased from 50 ppm (51.28 kJ/mol) to 100 ppm (54.89 kJ/mol). This is due to the physical barrier produced by the adsorbed molecules on the surface of carbon steel. This will increase the least energy required for corrosion reaction to occurs. It was observed that at both concentrations of 150 ppm and 200 ppm the activation energy of corrosion is 54.55 kJ/mol and 54.16 kJ/mol respectively which showed a downward trend compared to 100 ppm concentration but this is not lower in comparison to that of the blank solution. It has been reported that lower Ea value in the presence of inhibitors in comparison to the blank is attributed to chemical adsorption (Solomon et al., 2010; Thirumoolan et al., 2014; Alhaffar et al., 2018). Since the reverse is the case in this study it can be said that the mechanism of corrosion can be ascribed to physical adsorption (physisorption). At inhibitor concentration of 250 ppm the activation energy value was increased to 57.93 kJ/mol. The corrosion reaction will be pushed away on the surface site and occurs at the uncovered parts of carbon steel when inhibitor is added in hydrochloric acid solution thus giving a higher value of Ea (Quraishi et al., 2010).
The trend of increasing Ea values with inhibitor concentrations has been reported by other researchers on studies on various plant extract such as jujube leaves (Sivakumar and Mohana, 2012), black pepper (Quraishi et al., 2010), sunflower leaves (Hui et al., 2012) and piper nigrum extract (Norzila and Ishak, 2015). In this study, the increasing values of Ea obviously showed a physical adsorption of inhibitor molecules on the surface carbon steel. Physical adsorption happens due to the electrostatic force between negatively charged metal surface and positive charged of organic species (Norzila and Ishak, 2015).
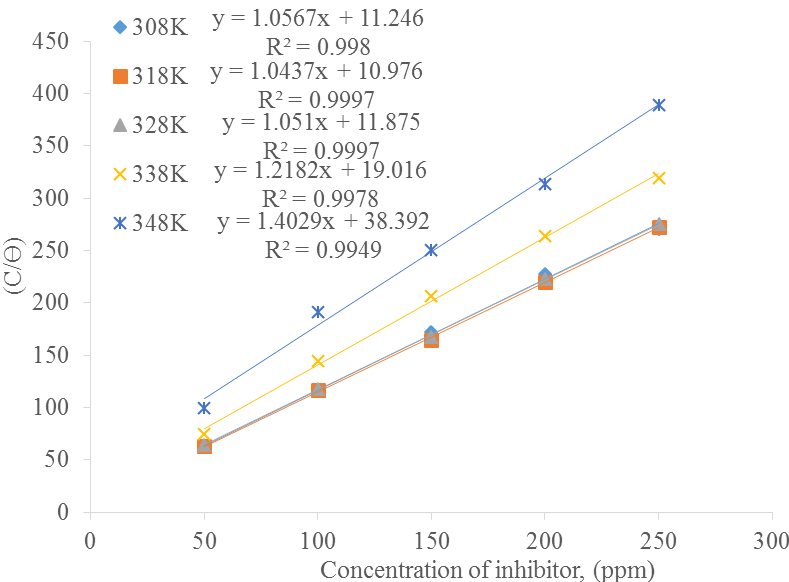
Adsorption isotherms: Four different adsorption isotherms were tested so as to obtain more knowledge about the interface between the carbon steel surface and inhibitor. The isotherms tested are Langmuir, Freundlich, Temkin and El-Awady adsorption isotherms. The linear regression coefficient of determination (R2) was used to adjudge the model that best fit the experimental values.
Langmuir relationship is represented by Eq. (6):
where, K is the equilibrium constant of adsorption (M-1) which was employed to obtain the Gibb’s free energy, C is the inhibitor concentration ppm and
where,
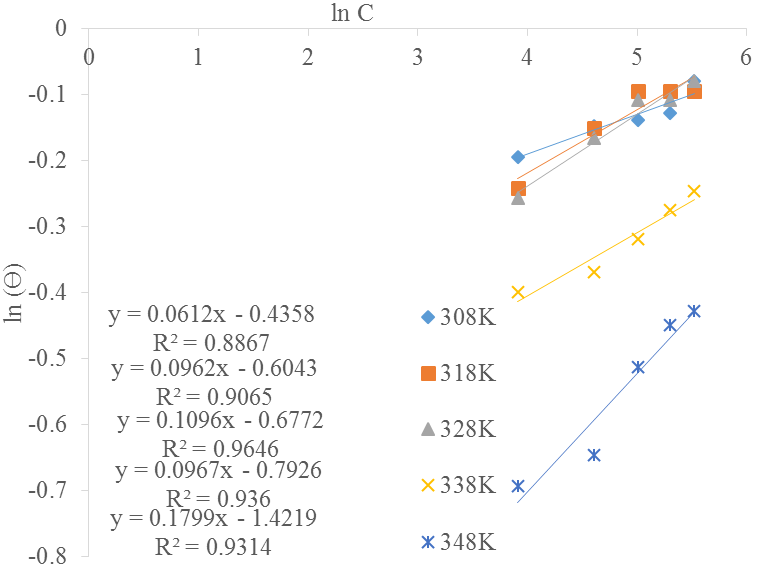
Freundlich isotherm: The fitting of non-ideal system can be done sometimes by fitting the experimental data to Freundlich adsorption isotherm as in Fig. 12 (Yaro and Khadom, 2008). This is expressed in Eq. (8):
Equation 8 can be re-written as:
A plot of
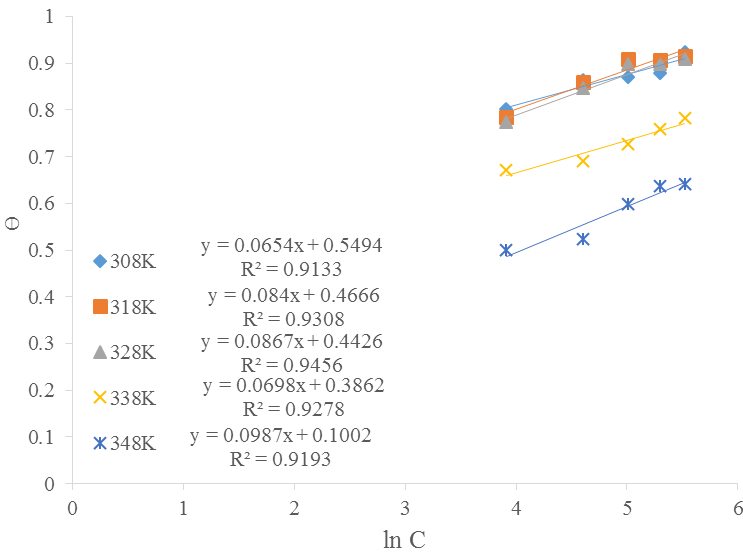
Temkin isotherm: This is expressed in Eq. (10):
Equation 10 can be re-written as:
where,
A plot of
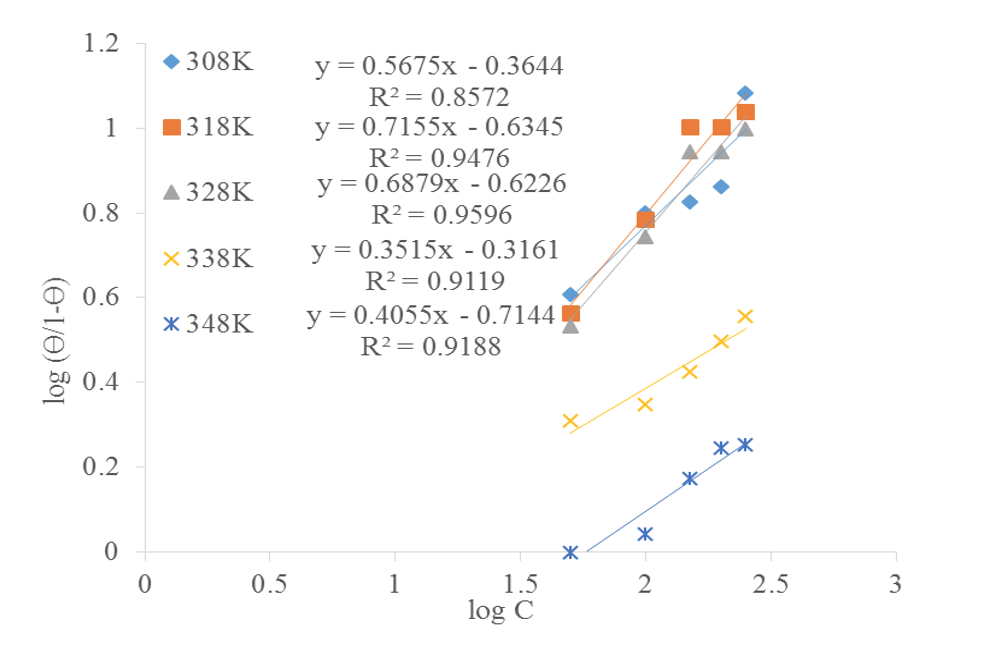
El-Awady isotherm: This model is also referred to as the kinetic/thermodynamic model and is written as follows:
where,
A plot of
When 1/y>1, each inhibitor molecule is believed to occupying more than one active site on the metal surface and vice-versa (Fouda and Ellithy, 2009).
Based on Table 4 to 7, the
Table 6 gives the Temkin isotherm model parameters where the effect of indirect adsorbate (inhibitor)/adsorbate (inhibitor) interactions with the metal surface on the adsorption process was confirmed. Generally, the coefficient of inhomogeneity (f) decreases except at 338 K. The Gibb’s free energy of adsorption
From Table 7, the value of
From the values of
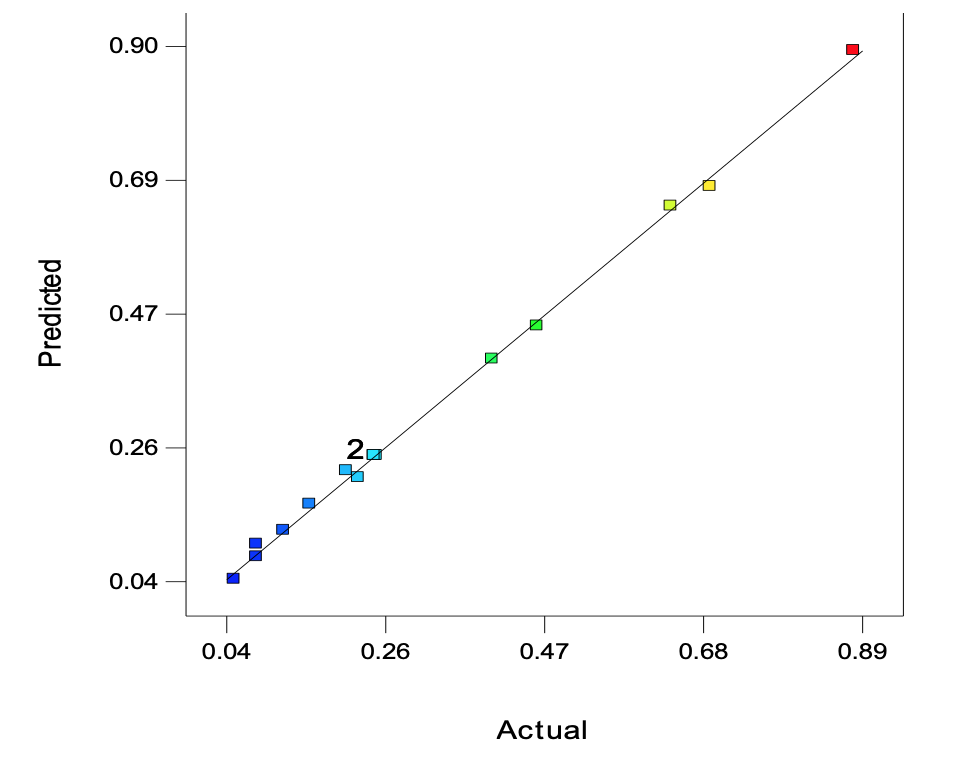
Optimization of corrosion process on carbon steel: Table 8 shows the experimental data for the predicted, observed and residual values obtained, the small values of the residual indicate that the data from the experiment fitted well to the quadratic model used. Also, results of the Analysis of Variance (ANOVA) as presented in Table 9 showed the model used was significant with F-value of 519.78 and with a corresponding low p-value
For the model to be adjudged to be of good fit R2 value should be at least 0.80 (Guan and Yao, 2008). The coefficient of determination
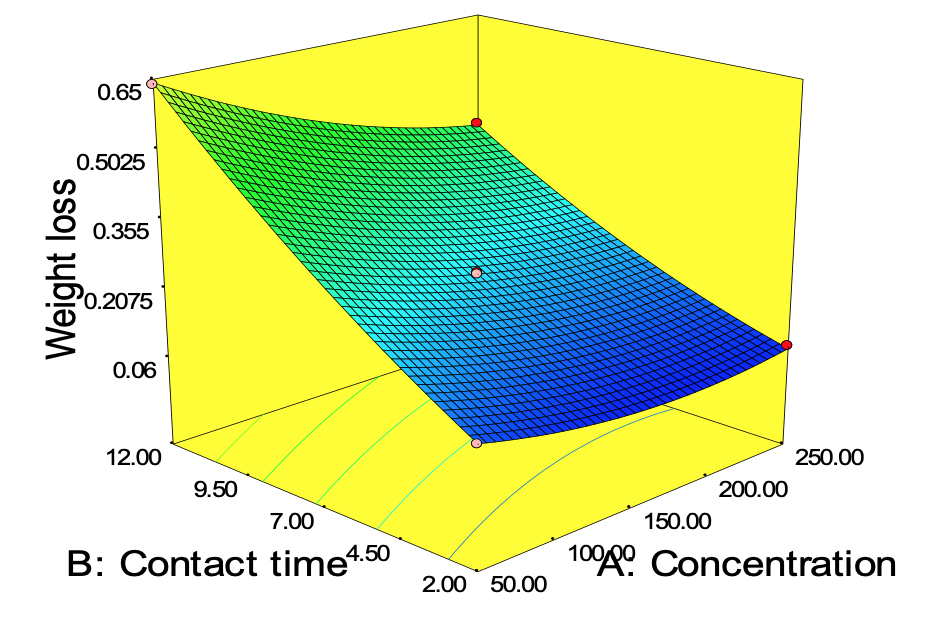
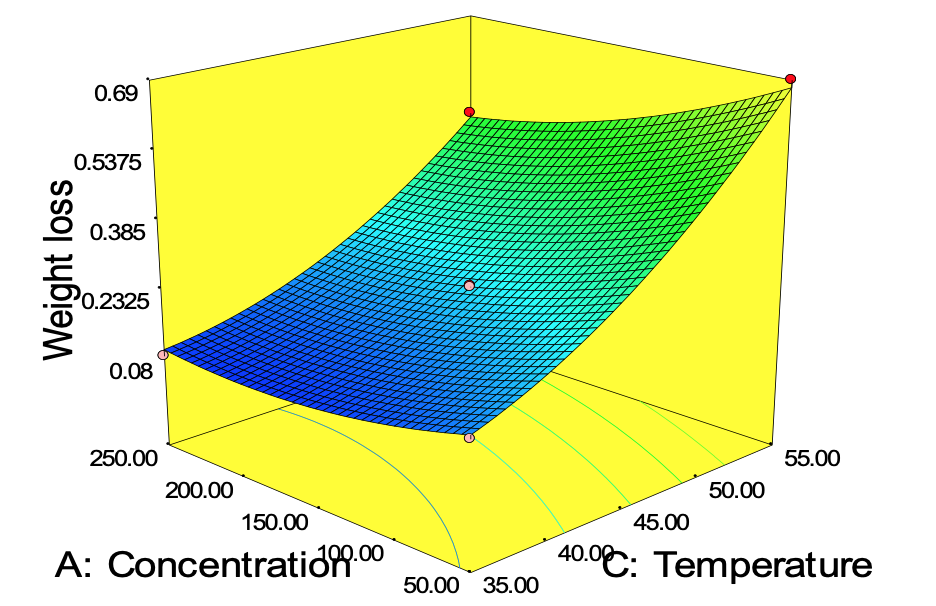
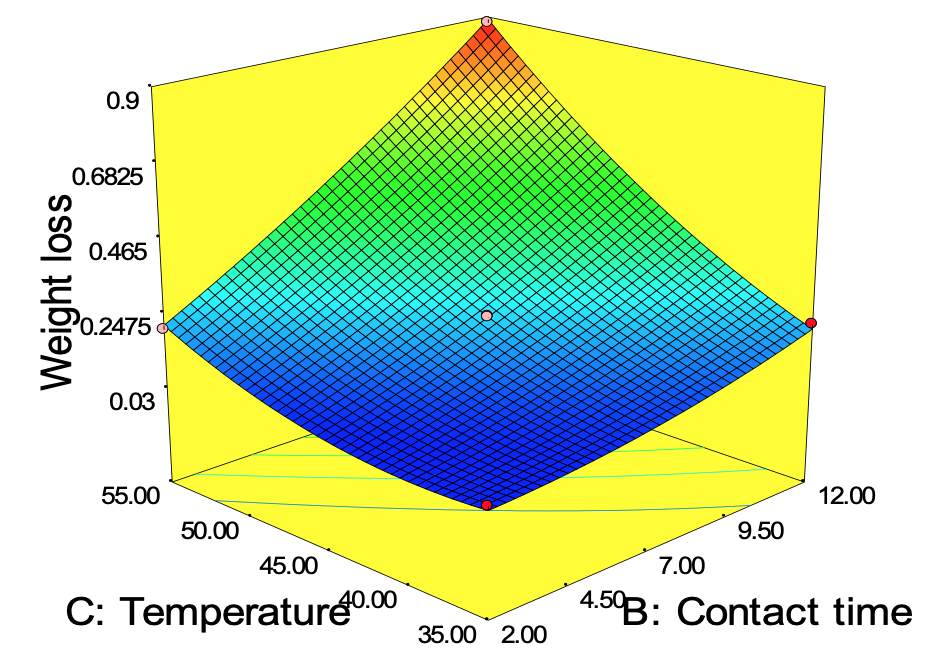
Figure 11 to 13 describes the surface plot for the weight loss of carbon steel corrosion process using Okro leaf extract as inhibitor. It was used to give an important evidence on the system behaviour within the experimental design on the corrosion of carbon steel using Okro leaf as an inhibitor. The different shapes of the contour plots indicated different interactions between the variables. An elliptical contour plot indicated interactions between the variables were significant while a circular contour plot means otherwise (Zhao et al., 2012). It can be seen in Fig. 11 that as concentration increases with contact time the weight loss of carbon steel decreases.
This can be as a result of higher adsorption of active inhibitor molecules from the extract on the metal surface forming a thin film on the metal surface thus preventing further attack from the corrosive environment. This in turn reduced the weight loss by specimen, which agrees with the findings of Yawas (2005) and Nwabanne and Okafor (2011). Figure 12 shows the interaction of concentration, temperature and weight loss. It can be deduced that the protective films of the inhibitor formed on the surface of the carbon steel are less stable at higher temperature thus leading to reduced weight loss at higher temperature. This may be due to desorption of some adsorbed molecules from the carbon steel surface at higher temperature thereby making the greater area of carbon steel to be exposed to attack from the acidic environment. Figure 13 revealed that at lower temperature and contact time, the weight loss of the carbon steel reduces. The Optimization tool of Design Expert 7.00 was used for the optimization. The goal of the optimization is to minimize the weight loss of carbon steel using the constraints shown in Table 11. The predicted optimal condition was; 198.71 ppm 2.04 h and 40.29°C for the concentration of inhibitor, contact time, temperature respectively while 0.045 g was the least weight loss predicted. The predicted optimum condition of the model was validated by conducting three replicates of experiments in the laboratory, 0.051 g weight loss of carbon steel was obtained which is in the range of the predicted value by the model equation.
| Chemical components | Plant extracts |
|---|---|
| Tannins | +++ |
| Steriods | +++ |
| Flavonoids | +++ |
| Saponins | + |
| Alkaloids | + |
| Anthraquinones | + |
| Phenols | +++ |
| Resin | ++ |
| Terpenoids | +++ |
| Cardiac Glycosides | +++ |
| +++ = Rich, ++ Moderate, + = good, - = Absent, Dheba et al., 2017 | |
| Concentration of Inhibitor | Ea (kJ/mol) | ∆H° (kJ/mol) | ∆S° (J/mol/K) |
|---|---|---|---|
| Blank | 25.62 | 22.89 | -131.29 |
| 50 ppm | 51.27 | 42.94 | -80.92 |
| 100 ppm | 54.89 | 48.09 | -68.09 |
| 150 ppm | 54.55 | 50.47 | -61.86 |
| 200 ppm | 54.16 | 50.97 | -61.00 |
| 250 ppm | 57.93 | 55.69 | -47.28 |
| Temperature, (K) | Kads | R2 | ∆G (KJ/mol) |
|---|---|---|---|
| 308 | 0.088 | 0.998 | -4.061 |
| 318 | 0.091 | 0.9997 | -4.281 |
| 328 | 0.084 | 0.9997 | -4.198 |
| 338 | 0.053 | 0.9978 | -3.01 |
| 348 | 0.026 | 0.9949 | -1.061 |
| Temperature, (K) | Kads | R2 | n | ∆G (KJ/mol) |
|---|---|---|---|---|
| 308 | 0.647 | 0.8867 | 0.0612 | -9.17 |
| 318 | 0.546 | 0.9065 | 0.0962 | -9.02 |
| 328 | 0.508 | 0.9646 | 0.1096 | -9.11 |
| 338 | 0.453 | 0.967 | 0.0967 | -9.06 |
| 348 | 0.241 | 0.9314 | 0.1799 | -7.50 |
| Temperature, (K) | Kads | R2 | f | ∆G (KJ/mol) |
|---|---|---|---|---|
| 308 | 4,448.5 | 0.9133 | 15.29 | -31.79 |
| 318 | 257.89 | 0.9308 | 11.90 | -25.29 |
| 328 | 164.54 | 0.9456 | 11.53 | -24.87 |
| 338 | 253.22 | 0.9278 | 14.33 | -26.84 |
| 348 | 2.76 | 0.9193 | 10.13 | -14.55 |
| Temperature, (K) | Kads | R2 | y | 1/y | B | ∆G (KJ/mol) |
|---|---|---|---|---|---|---|
| 308 | 0.4321 | 0.8572 | 0.5675 | 1.762 | 0.23 | -8.140 |
| 318 | 0.232 | 0.9476 | 0.7155 | 1.398 | 0.129 | -6.76 |
| 328 | 0.238 | 0.9596 | 0.6879 | 1.454 | 0.124 | -7.04 |
| 338 | 0.483 | 0.9119 | 0.3515 | 2.845 | 0.126 | -9.24 |
| 348 | 0.393 | 0.9188 | 0.4055 | 2.466 | 0.099 | -8.92 |
| Run order | X1, (ppm) | X2, (Hr) | X3, (°C) | Actual value | Predicted values | Residual | |
|---|---|---|---|---|---|---|---|
| 1 | -1 | +1 | 0 | 0.638 | 0.64 | -0.00475 | |
| 2 | 0 | +1 | -1 | 0.22 | 0.21 | 0.014 | |
| 3 | 0 | 0 | 0 | 0.244 | 0.24 | 0.0018 | |
| 4 | 0 | 0 | 0 | 0.243 | 0.24 | 0.0008 | |
| 5 | +1 | +1 | 0 | 0.399 | 0.4 | 0.002 | |
| 6 | 0 | 0 | 0 | 0.241 | 0.24 | -0.0012 | |
| 7 | +1 | -1 | 0 | 00.084 | 0.079 | 0.00475 | |
| 8 | 0 | +1 | +1 | 0.882 | 0.89 | -0.011 | |
| 9 | 0 | -1 | 0 | 0.054 | 0.043 | 0.011 | |
| 10 | 0 | -1 | +1 | 0.204 | 0.22 | -0.014 | |
| 11 | -1 | -1 | 0 | 0.12 | 0.12 | -0.002 | |
| 12 | +1 | 0 | 0 | 0.084 | 0.1 | -0.016 | |
| 13 | +1 | 0 | +1 | 0.459 | 0.45 | 0.008875 | |
| 14 | -1 | 0 | +1 | 0.69 | 0.67 | 0.016 | |
| 15 | 0 | 0 | 0 | 0.241 | 0.24 | -0.0012 | |
| 16 | 0 | 0 | 0 | 0.242 | 0.24 | -0.0002 | |
| 17 | -1 | 0 | -1 | 0.155 | 0.16 | -0.008875 |
| Sources | Sum of squares | Df | Mean square | F-values | Prob>F, p-value |
|---|---|---|---|---|---|
| RSM model | 0.88 | 9 | 0.098 | 519.78 | <0.0001 Significant |
| Residual | 0.001314 | 7 | 0.0001876 | ||
| Lack of fit | 0.001307 | 3 | 0.0004356 | 256.23 | <0.0001 Significant |
| Pure error | 0.0000068 | 4 | 0.0000017 | ||
| Cor total | 0.88 | 16 | |||
| CR-squared | 0.9985 | ||||
| Predicted R-squared | 0.9762 | ||||
| Adjusted R-squared | 0.9966 | ||||
| Adequate precision | 80.880 |
| Sources | Sum of squares | Df | Mean square | F-values | Prob>F, p-value |
|---|---|---|---|---|---|
| X1 | 0.042 | 1 | 0.042 | 221.78 | <0.0001 |
| X2 | 0.35 | 1 | 0.35 | 1873.39 | <0.0001 |
| X3 | 0.37 | 1 | 0.37 | 1975.28 | <0.0001 |
| X1X2 | 0.01 | 1 | 0.01 | 54.90 | 0.0001 |
| X1X3 | 0.0064 | 1 | 0.0064 | 34.11 | 0.0006 |
| X2X3 | 0.066 | 1 | 0.066 | 349.25 | <0.0001 |
| X12 | 0.005929 | 1 | 0.005929 | 31.60 | 0.0008 |
| X22 | 0.0003923 | 1 | 0.003923 | 20.91 | 0.0026 |
| X32 | 0.019 | 1 | 0.019 | 101.55 | <0.0001 |
| Constraints | Goal | Lower limit | Upper limit |
|---|---|---|---|
| Concentration of inhibitor | In range | 50 | 250 |
| Contact time | In range | 2 | 12 |
| Temperature | In range | 35 | 55 |
Conclusion
In the present study, Okro leaf was extracted and characterized using phytochemical test and was used as an inhibitor. The effects of corrosion inhibition of the Okro leaf extract for carbon steel in 1.5M HCl solution was estimated using the gravimetric technique. Thermodynamic, adsorption and optimization studies were also carried out to estimate the binding/adsorption mechanism of the inhibitor molecules on the surface of the carbon steel. The corrosion rate decreased as inhibitor concentration is increased. The corrosion inhibition performance of Okro leaf extract is found to depend on its concentration and temperature. Corrosion inhibition of the Okro leaf extract on carbon steel surface occurred by the physical adsorption mechanism. This was evidenced with the values of activation energy obtained in the inhibited medium which is higher than that obtained in the blank solution. In addition, the rate of corrosion was maximum in the blank solution since it is devoid of inhibitor that can impede the corrosion deterioration process. The effects of inhibitor concentration, contact time and temperature on weight loss of carbon steel is carried out using Response Surface Methodology (RSM). It was established that all the variables considered are significant factors on the corrosion inhibition process. Second-order mathematical model fitted well to the experimental data obtained. The optimum condition that was established were; 198.71 ppm 2.04 h and 40.29°C for the concentration of inhibitor, contact time, temperature respectively. The activation enthalpy of the corrosion process increases from 22.89 kJ/mol to 55.69 kJ/mol. The positive values of the enthalpy of the corrosion system signified an endothermic adsorption process. The activation entropy (∆S°) values in the absence and presence of inhibitor were all negatives. This implied to mean that the activated complex in the rate - determining step represents association rather than dissociation step, indicating that during the adsorption process, a decrease in the degree of orderliness takes place. Experimental data fitted well to Langmuir, Freundlich and El-Awady isotherms. However, mixed type adsorption mechanisms were confirmed on corrosion inhibition of Okro leaf extract on the carbon steel surface. The adsorption process is spontaneous with the negative value of
Conflict of Interest
This research article is an original work of the authors and was carried out by the authors. The authors declare no conflict of interest in whatsoever form.
Author Details
1Department of Chemical Engineering, Federal University of Petroleum Resources,
2Department of Petroleum and Natural Gas Processing Engineering, Petroleum Training Institute, Effurun, Delta State, Nigeria
References
Rights and permissions
Open Access: This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third-party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
Cite this Article
DOI: http://doi.org/10.19026/rjaset.17.6038
Sections
