Advance Journal of Food Science and Technology
Angiotensin Converting Enzyme Inhibition by Aqueous Extract of Citrus sudachi Peels
Advance Journal of Food Science and Technology 2019 17: 37-42
Cite This ArticleAbstract
The effect of the aqueous extract of sudachi (Citrus sudachi) peels on Angiotensin-I Converting Enzyme (ACE) activity was examined in vitro to assess its potential ability to improve high blood pressure. The obtained results indicated that the extract caused the concentration-dependent inhibition of the enzyme activity in a competitive manner and suggested that putative active substances might be heat-stable with dialyzable molecular size. By comparison with the extracts prepared from lemon and orange peels, the extract of sudachi peels was shown to have the most potent inhibitory activity, which might be closely related to the amounts of total polyphenolic compounds in these extracts. The contents of phenolic acids and flavonoid compounds in the extract of sudachi peels were also shown to be higher than those of two other extracts. Thus, it seems possible to consider that the aqueous extract of sudachi peels may contain substantial amounts of water soluble polyphenolic compounds responsible for its inhibitory action on the enzyme.
Keywords:
Introduction
Hypertension is a typical pathologic condition of lifestyle-related diseases and known as a risk factor of cardiovascular disease. One of the causes of hypertension is considered to be attributed to an increase in volume of body fluid in the circulatory system caused primarily by chronic failure in the renal function. In this case, the rennin-angiotensin-aldosterone system is considered to play a central role in the elevation of blood pressure. In general, Angiotensin-I Converting Enzyme (ACE) is known as the enzyme catalyzing the conversion of inactive angiotensin-I to active angiotensin-II, resulting in the elevation of blood pressure. Thus, the inhibition of ACE activity is considered to reduce the blood pressure and can improve the physical condition of hypertensive patients. Hence, it seems meaningful to search for anti-hypertensive substances in daily foods to make the day-to-day management of blood pressure much easier and safer. For this purpose, many efforts have been made to find out potentially active substances inhibiting the production of hypertensive peptide angiotensin II in a variety of natural food materials, such as various plant foods including wheat, rice, peas, vegetables and fruits, which have been enthusiastically evaluated by investigating their inhibitory actions on the ACE activity in vitro. (Martinez-Maqueda et al., 2012; Medina-Remnón et al., 2013). From these lively researches, many active peptides inhibiting ACE activity have been identified in the extracts prepared from cow pea, Korean rice wine and seaweed (Suetsuna et al., 2004; Segura et al., 2010; Kang et al., 2012).
Polyphenolic compounds in plant foods, on the other hand, have been attracted attention by its antioxidant properties and evidence for suggesting their functions in the prevention of cardiovascular disease and cancer has recently been gathered. In addition, polyphenolic compounds in foods and beverages, such as chocolates, wine and tea, have also been suggested to play a potential role in reducing the blood pressure through the inhibition of ACE activity, thereby exhibiting the beneficial effect on high blood pressure (Actis-Goretta et al., 2003; Actis-Goretta et al., 2006; Dong et al., 2011; Oboh and Ademosun, 2011a; Oboh and Ademosun, 2011b; Guerrero et al., 2012; Al-Shukor et al., 2013). Plant foods, particularly fruits and vegetables, are recognized as a major source of polyphenolic compounds and the processing of these foodstuffs is considered to produce the by-products containing a large amount of polyphenolic compounds, which can be regarded as valuable source of polyphenolic compounds. In particular, citrus fruits are generally known as one kind of typical polyphenol-rich fruits and a wide variety of citrus fruits are cultivated in many place around the world. Among them, sudachi is a sour citrus used popularly in Japan as a seasoning and flavoring and characterized by its relatively high acidity and pleasant refreshing aroma. As its juice is used in most of the time, the residual peels are discarded in spite of containing various and abundant polyphenolic compounds. Therefore, it seems quite reasonable to expect that sudachi peels may have various biological activities based on polyphenolic compounds contained in the peels. For example, since polyphenolic compounds, phenolic acid, flavonoids and other compounds, in citrus peels are well known to have the antioxidant activity and also suggested to improve high blood pressure, sudachi peels may possibly be predicted to have a potential activity to modulate the function of renin-angiotensin-aldosterone system (Galleano et al., 2010; Hügel et al., 2016; Al-Shukor et al., 2013). In the present study, the aqueous extract of sudachi peels was prepared by homogenizing the peels in water and the effect of this extract on ACE activity was examined in vitro.
Materials And Methods
Preparation of peel extracts: Fresh fruits of Citrus sudachi were purchased from a local market and obtained peels were homogenized with water at the concentration of 5 g of peels in 100 mL and the homogenate was centrifuged at 12000×g for 20 min. Obtained supernatant fractions were filtered through a syringe-top disk filter (0.2 μm) and then stored at -20°C until use. Citrus lemon and Citrusorange peels were also used as a reference sample.
Determination of ACE activity: ACE activity was measured as described before (Mallikarjun Gouda et al., 2006; Nishibori et al., 2012; Nishibori et al., 2013a; Nishibori et al., 2013b). Briefly, the mixture containing 0.1 M borate buffer (pH 8.0), 1 M NaCl, 1 mM HHL, 0.5 mU ACE and 100 μL of the extracts in total volume of 150 μL was incubated at 37°C for 60 min and the reaction was terminated by adding 10 μL of 5 M hydrochloric acid (HCl). The amount of hippuric acid formed during the reaction was determined by a HPLC analysis using C18 column (CAPCELPAK ACR 1.5×150 mm) and 20% MeOH containing 0.1% trifluoloacetic acid as an eluent at a flow rate of 0.15 mL/min and detected at 230 nm.
Determination of phenolic compounds: Phenolic compounds in these extracts were determined using Folin-Ciocalteu phenol reagent according to the method described in Chen et al. (2007). Briefly, the diluted extracts (100 μL) were mixed with 400 μL of Folin-Ciocalteu phenol reagent (diluted at 10-fold with water) and then added 500 μl of 5% sodium carbonate solution and the mixture was kept for 20 min at room temperature. The absorbance at 765 nm was measured and total polyphenol amounts were expressed as an equivalent to gallic acid.
Determination of phenolic acids: Free phenolic acids were isolated and determined as described by Subba Rao and Muralikrishna (2002). Briefly, the aqueous extracts were adjusted to pH 2-3 with 4 M HCl and phenolic acids were transferred to the organic solvent by ethyl acetate extraction (5 times). The pooled organic fractions were then treated with anhydrous disodium sulfate to remove moisture and filtered to remove the salt and then evaporated to dryness. The residue was dissolved in methanol and phenolic acid amounts were determined using Folin-Ciocalteu phenol reagent as described above.
Determination of flavonoids: Flavonoid contents in the extracts were measured using an aluminum chloride colorimetric method (Chang et al., 2002). Briefly, 100 µL of the extract was mixed with 300 µL of methanol, 20 µL of 10% aluminum chloride, 20 µL of 1 M potassium acetate and 560 µL of distilled water and the mixture was kept for 30 min at room temperature and the absorbance at 415 nm was then determined. The contents of flavonoid compounds in the extracts were expressed as an equivalent to quercetin.
Statistical analysis: Results were presented as the mean±SEM and the statistical analyses were then carried out using a one-way Analysis of Variance (ANOVA) followed by Tukey’s post hoc test. The difference between two values with p<0.05 was regarded as indicating a statistically significant.
Results And Discussion
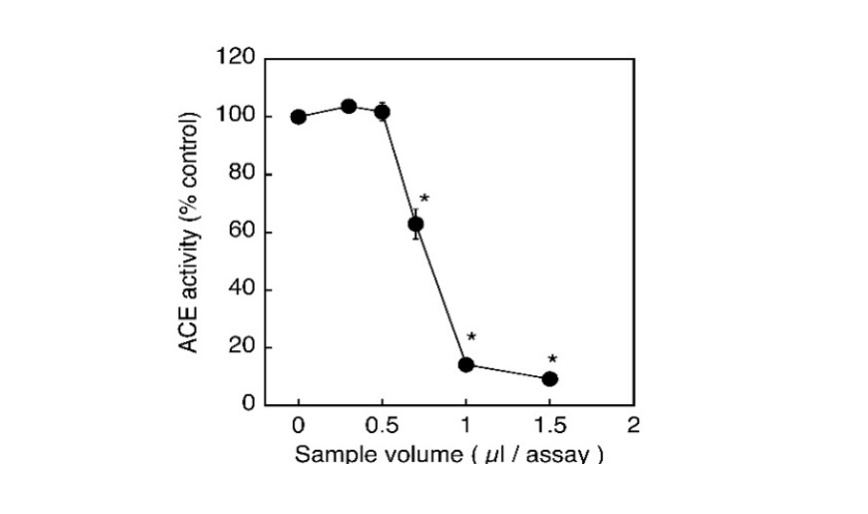
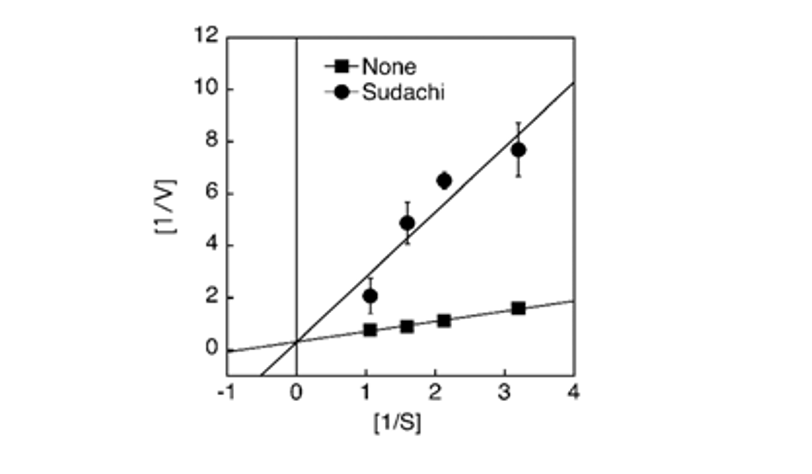
The biologically active peptide angiotensin II, known to be involved in the elevation of blood pressure, is produced by the conversion of inactive angiotensin-I catalyzed by Angiotensin-I Converting Enzyme (ACE). Therefore, the inhibition of ACE activity is recognized to result in the reduction of blood pressure, thereby being considered to be one of the effective measures to improve the conditions of patients with hypertension. In the present study, the aqueous extract of sudachi peels was shown toinhibit the ACE activity in vitro in a concentration-dependent manner and the enzyme activity was suppressed up to approximately 10% of the control at 1 μL addition of 5% sudachi peel extract (Fig. 1). To characterize the inhibitory action of sudachi peel extract on the ACE activity, the kinetic properties of the enzyme inhibition were then examined using a Lineweaver-Burk plot analysis. Consequently, the aqueous extract of sudachi peels was shown to cause the competitive inhibition of ACE activity under the assay conditions used here (Fig. 2).
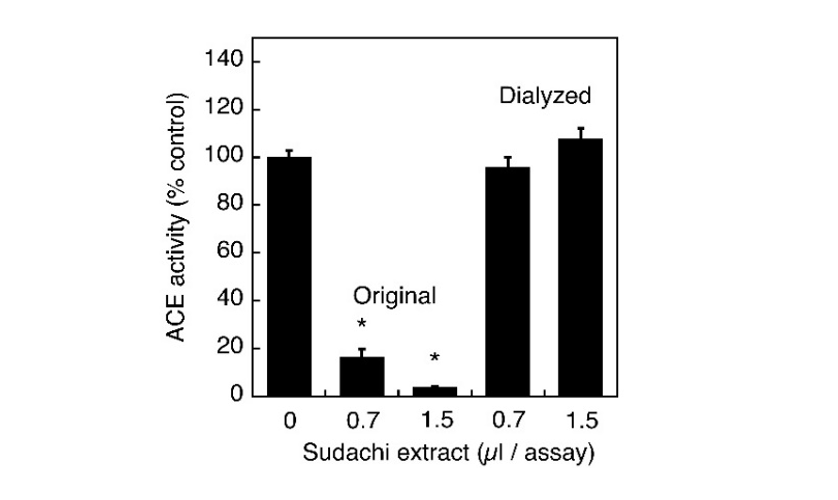
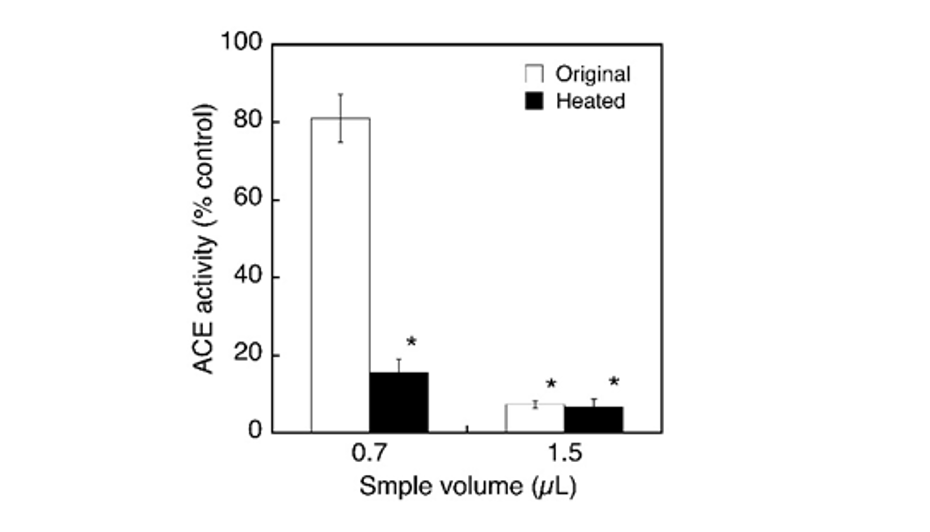
Previously, many substances, such as polyphenols and short peptides derived from various foods and food stuffs have been reported to cause the ACE inhibitory activities and most of them have also been shown to inhibit this enzyme in a competitive manner. So, it seems meaningful to characterize the properties of putative ACE inhibitory substances in the extract of sudachi peels. First, the extract was subjected to dialysis for overnight against distilled water and the inhibitory action of dialyzed extract on ACE activity was examined to estimate roughly the molecular size (s) of inhibitory substance (s) in the extract. Because the inhibitory activity of the dialyzed extract was markedly reduced by dialysis, putative inhibitory substances might be removed during dialysis and therefore could be speculated to have a relatively small molecular size (Fig. 3). To further characterize putative active substances contained in the aqueous extract of sudachi peels, the extract was autoclaved at 121°C for 30 min and the ACE inhibitory activity was then determined after heat treatment. The ACE inhibitory activity of heated extract was almost identical to that of the original extract at 1.5 µL addition, or rather might be more potent at 0.7 µL addition, although the reason was not elucidated yet (Fig. 4). Thus, it seemed possible to consider that the ACE inhibitory substances in sudachi peels might be autoclave stable.
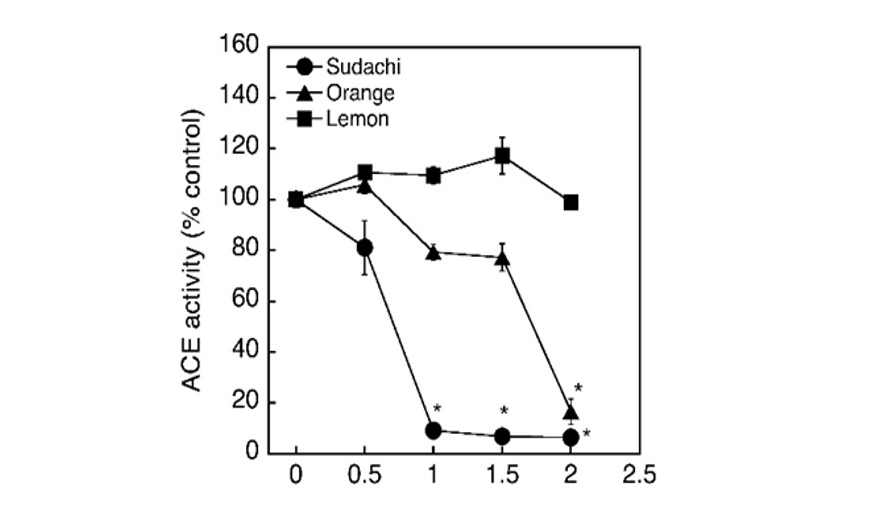
Citrus peels, such as shaddock and grapefluit peels, are well known to have the ability to inhibit ACE activity and the active substances are shown to be organic solvent-extractable. On the other hand, we detected the ACE inhibitory activity in the aqueous extract of sudachi peels and suggested that the inhibitory substances contained in sudachi peels was water-soluble and therefore presumed that the inhibitory substances may possibly be distributed in citrus peels other than sudachi peels. Then, the aqueous extracts were prepared from the peels of orange and lemon as same as sudachi peels and the inhibitory activities of these extracts on the enzyme activity were tested. Consequently, sudachi peel extract showed the most potent activity among three extracts tested and the inhibitory action of sudachi peel extract was approximately 2-times as potent as that of the orange peel extract. However, the lemon peel extract failed to show any significant effect at the concentrations tested (Fig. 5). Thus, the aqueous extract of sudachi peels was shown to have the highest enzyme inhibitory activity among these citrus peels extracts examined in the present study. Polyphenolic compounds derived from food and foodstuff have been established to have the antioxidant and radical scavenging activities and are generally considered to be one of the major inhibitory substances of ACE activity (Liu et al., 2003; Actis-Goretta et al., 2006; Ahmed et al., 2010; Liu et al., 2010; Dong et al., 2011; Al-Shukor et al., 2013). Therefore, it seemed meaningful to analyze the amounts of polyphenolic compounds contained in these extracts to obtain further information about the putative substances causing the ACE inhibition.
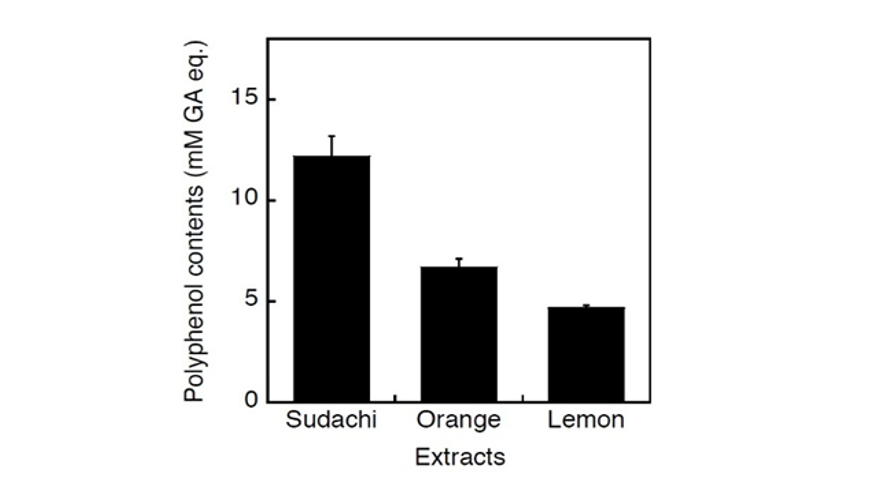
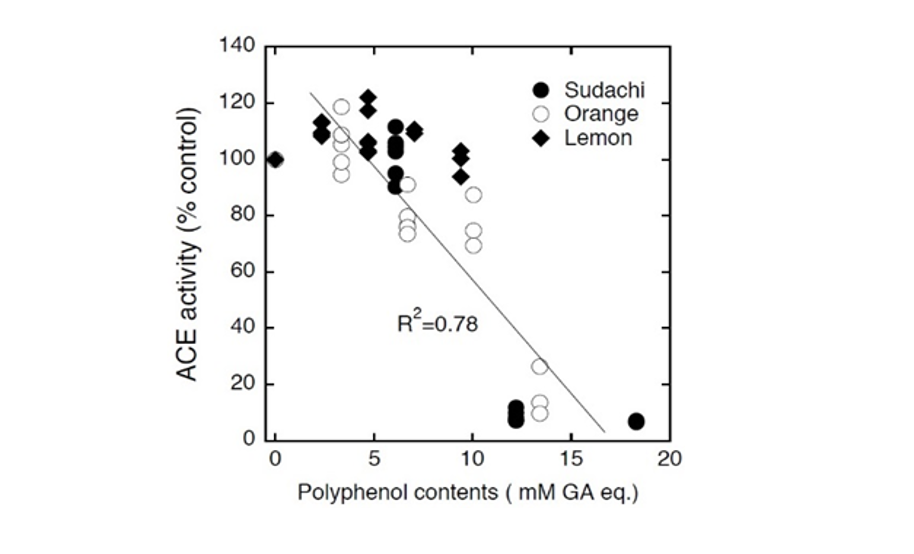
Consequently, the concentration of polyphenolic compounds in sudachi extract was shown to be highest among these three extracts and lowest in lemon extract (Fig. 6). Thus, these polyphenolic contents could be considered to correspond to ACE inhibitory activities of these citrus extracts. In fact, the contents of polyphenolic compounds in these extracts were shown to be closely related to their ACE inhibitory activities (Fig. 7) and therefore it seemed possible to speculate that potential ACE inhibitory substances in sudachi peel extract might be water-soluble polyphenolic compounds. Thus, it seemed reasonable to consider that heat-stable and small molecular polyphenolic compounds might be major ACE inhibitory compounds in the aqueous extract of sudachi peels. On this point, polyphenolic compounds have previously been proposed as one of the major low molecular weight substances to inhibit ACE activity (Guerrero et al., 2012; Al-Shukor et al., 2013; Xu et al., 2013; Wunpathe et al., 2018). Then, HPLC analysis of sudachi peel extract was performed to confirm that the extract practically contained the substantial amounts of polyphenolic compounds according to the assay method reported previously (Safdar et al., 2017). Consequently, obtained chromatograms gave us many peaks (over 15 major peaks) and indicated evidently that the aqueous extract of sudachi peels contained various phenolic acids and flavonoids (data not shown). In addition, the contents of polyphenolic compounds in the aqueous extracts of three citrus peels were also determined and the concentrations of phenolic acids and flavonoids in the extract of sudachi peels were shown to be much higher than those of orange and lemon peel extract (Table 1). These findings seemed to provide further evidence for supporting the conclusion that phenolic acid and flavonoids in the sudachi peel extract might be major components responsible for its ACE inhibitory action observed here and sudachi peels are therefore considered as a good resource of water soluble ACE inhibitory substance.
| Phenolic acid (mM) | Flavonoid (mM) | |
|---|---|---|
| Sudachi | 9.6 | 0.9 |
| Orange | 7.1 | 0.3 |
| Lemon | 5.2 | 0.3 |
Conclusion
In the present study, the aqueous extract of sudachi peels was shown to inhibit the ACE activity in a competitive manner and suggested that putative active substances in the extract might be heat-stable with dialyzable molecular size. By comparison with the extracts of lemon and orange peels, the contents of phenolic acids and flavonoid compounds in the extract of sudachi peels were shown to be higher than those in the orange and lemon peel extracts and the sudachi peel extract was shown to have the most potent inhibitory activity, which might be closely related to the amounts of total polyphenolic compounds contained in the extracts. Thus, it seems possible to consider that the aqueous extract of sudachi peels may contain the substantial amounts of polyphenolic compounds responsible for its inhibitory action on the ACE activity.
Author Details
1Department of Food Science and Nutrition, Shikoku Junior College,
2Department of Nursing,
3Life Science Research Group, Shikoku University, Ohjin, Tokushima 771-1192, Japan
References
Rights and permissions
Open Access: This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third-party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
Cite this Article
DOI: http://doi.org/10.19026/ajfst.17.6010
Sections
